Monk Fruit and Stevia
Is monk fruit sweetener healthier than stevia sweetener? Sweeteners from monk fruit and stevia share many similarities in both chemistry and functionality. The future of those sweeteners lies in effective natural bitterness masking agents
3/13/20243 min read
A company selling plant-based protein powder puts eye-catching word " No Stevia" on the package yet uses monk fruit extract to sweeten the product. This company is not alone, many similar protein companies follow the same marketing strategy. People began to make prediction that monk fruit extract will replace stevia extract as the top natural zero-calorie sweetener. Perhaps they are unaware that Johnson & Johnson's subsidiary McNeil Nutritionals launched monk fruit extract based sweetener in 2012 and discontinued it only two years later due to disappointing sales.
While the idea of using fruit sounds healthier than leaf, the question remains: Is monk fruit extract truly a healthier sweetener than stevia extract? Unfortunately, there is no scientific evidence supporting this claim. Let’s avoid getting caught up in trace component differences; at the low usage levels, those trace compounds contribute little to the health even if they do at much larger dosage.
Monk fruit sweeteners have witnessed significant growth in recent years, notably by the inclusion in Diet Coke’ limited version plant-based sweetener offering. The North American market dominates the monk fruit sweeteners industry, with the USA leading in consumption (true for stevia as well).
Monk fruit sweeteners are derived from the plant Siraitia grosvenorii native to Southern China, where is also its primary producer. Comparing to stevia, monk fruit has higher production cost and much less supplies, which makes the aforementioned predication extremely difficult, if not impossible, to come true. The recent rise of monk fruit may stem from the failure of stevia-based sweeteners to fully substitute sugar even after 15 years’ development. There is hope that monk fruit may not have the bitter aftertaste or alleviate the unpleasant aftertaste associated with stevia if used in combination. Is monk fruit really the sweetener of tomorrow?
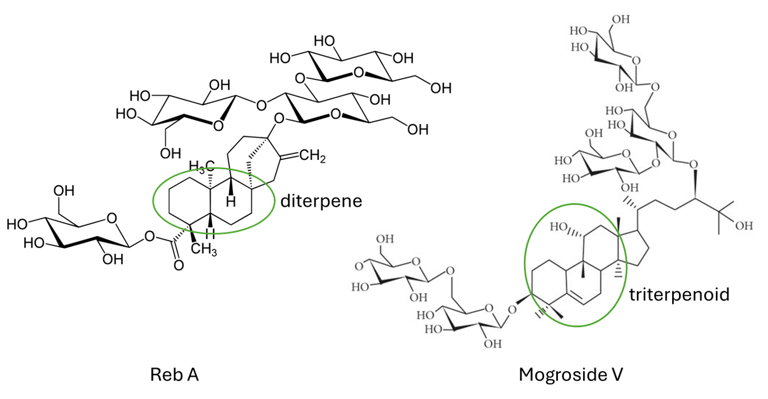
While reb A is the most abundant steviol glycoside in stevia leaf, mogroside V is the major sweet compound in monk fruit. Remarkably, despite their distinct origins, these compounds share a similar level of sweetness. Both reb A and mogroside V have terpenes as their structural backbone—one being a diterpene and the other a triterpene (as depicted in the image above), therefore both steviol glycoside and mogroside belong to natural terpene glycosides. Interestingly, the bitterness associated with stevia might be linked to the diterpene center, as many terpenes tend to have a bitter taste. It’s reasonable to conclude that mogroside V has a bitter taste as well. In fact, lingering bitter aftertaste is indeed a characteristic of monk fruit sweetener. There is abundant information available regarding their benefits and applications, in this post, we will delve into something different — not fancy taste receptors, but rather insights that are rarely found online.
Let’s start with chemistry. Reb A has 4 glucoses while mogroside V contains 5. There is a theory that the more glucose attached, the sweeter and less bitter the compound tends to be. It is confirmed by correlating the sweetness and bitterness of stevioside, reb A, reb D, and reb M with their glucose units. Encouraged by the correlation, the industry continues digging into stevia leaf and monk fruit, hoping to find a new molecule devoid of bitter aftertaste. Currently, stevia is leading the race, with monk fruit closely following suit. Nevertheless, many promises fell flat. And it comes with another challenge: solubility.
Reb M, the promising steviol glycoside, has poor water solubility which makes the formulation for concentrated beverage syrup very changeling. Mogroside V water solubility is worse than reb A (some literature claim it is easily soluble in water, likely refer to monk fruit extract with low mogroside V content). So it’s generally true that more glucose leads to lower solubility, although the correlation is not linear. But why does this occur, especially considering that glucose is highly hydrophilic?
The chemistry behind it is complex. A simpler explanation is dextrin versus cellulose. They are both polysaccharides, but dextrin is water solubility and cellulose is insoluble. The difference results from how the glucoses are linked with each other. Dextrin is linked through alpha glycosidic bonds, while cellulose uses beta linkage. All the naturally occurring steviol glycosides or mogrosides are beta glycosylation except one. On the other hand, enzyme modified stevia (EMS) or glycosylated steviol glycoside (GSG) adds new glucose by alpha linkage, none of those GSGs has been found in nature. EMS/GSG has improved solubility.
Based on this fundamental science analysis, the chance of finding a new molecule without bitter aftertaste in both stevia and monk fruit is slim. Furthermore, any such a new molecule may be accompanied by unintended low solubility that hinders its practical applications.
Monk fruit extract alone is not the sweetener of tomorrow. It is likely that Coke blends monk fruit with stevia extract (reb M) simply due to reb M's poor water solubility (Reb D is worse).
The future of stevia and monk fruit lies not in finding more and more new molecules, or in unfounded claims one is better than the other, but rather in identifying natural ingredients masking bitterness, improving upfront onset and reducing lingering sweetness that are proven to work effectively with reb M or mogroside V by end users.
